2021 China New Design Cisatracurium Besylate - Cholestyramine CAS 11041-12-6 USP Standard High Purity – Ruifu
2021 China New Design Cisatracurium Besylate - Cholestyramine CAS 11041-12-6 USP Standard High Purity – Ruifu Detail:
Supply with High Purity and Stable Quality
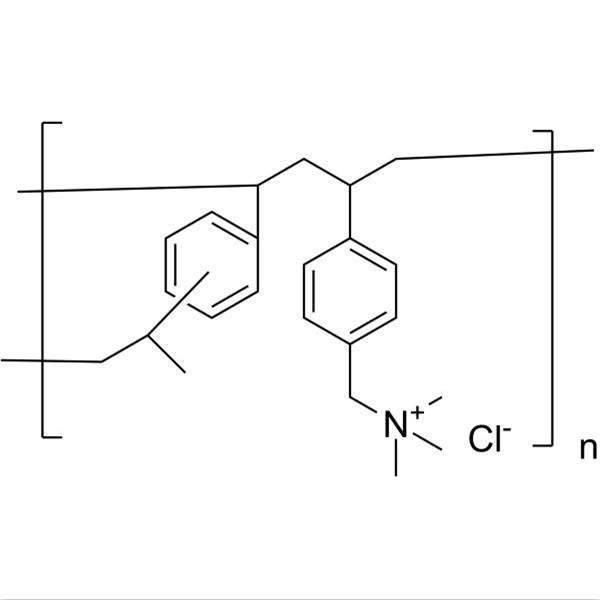
Chemical Name: Cholestyramine
Synonyms: Cholestyramine Resin; Colestyramine
CAS: 11041-12-6
Cholestyramine in the treatment of type Ⅱa hyperlipoproteinemia
API High Quality, Commercial Production
| Chemical Name | Cholestyramine |
| Synonyms | Cholestyramine Resin; Colestyramine |
| CAS Number | 11041-12-6 |
| CAT Number | RF-API23 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C27H47N |
| Molecular Weight | 385.66878 |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Powder, Odorless or has a slightly amine-like odor, Hygroscopic |
| Identification | The infrared absorption spectrum of the sample should be consistent with the reference standard |
| pH | 4.0~6.0 |
| Dialyzable Quaternary Amine | Should meet the requirements |
| Chloride | 13.0%~17.0% (Calculated on the dried basis) |
| Loss on Drying | ≤12.0% |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤20ppm |
| Bacterial Count | ≤1000 cfu/g |
| Yeast and Mold | ≤100 cfu/g |
| Escherichia Coli | Should not be detected |
| Live Mite Test | Should not be detected |
| Purity | Calculated on the dried basis, each g exchanges 2.0g~2.4g of Sodium Taurocholate |
| Test Standard | United States Pharmacopeia (USP) |
| Usage | Active Pharmaceutical Ingredient (API) |
Package: Bottle, Aluminum foil bag, Cardboard drum, 25kg/Drum, or according to customer’s requirement.
Storage Condition: Store in sealed containers at cool and dry place; Protect from light, moisture and pest infestation.


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Cholestyramine (CAS: 11041-12-6) with high quality, active pharmaceutical ingredient (API). Cholestyramine (Colestyramine) is a bile acid binding resin and can inhibit intestinal bile acid absorption which results in the increasing bile acid synthesis from cholesterol. A strongly basic anion exchange resin whose main constituent is polystyrene trimethylbenzylammonium Cl(-) anion.
Cholestyramine, is a strongly basic quaternary ammonium anion exchange resin, it is used in low-density lipoprotein hyperlipidemia that includesⅡa, Ⅱb hyperlipidemia,it is the strongest cholesterol-lowering drug. After oral administration, it is not absorbed, in the intestine the containing chlorine ion is exchanged with the bile acid, it can bind stably with bile acids until the feces, it can reduce the absorption of exogenous cholesterol, hinder the enterohepatic circulation of bile acids absorbed into blood, it can reduce the amount of bile acids in the blood, which prompt blood cholesterol conversion to bile acids, which lowers cholesterol. Cholestyramine has no lowering effect on triglycerides. Because of the powerful cholesterol-lowering effect, the product becomes the choice of drug for type Ⅱa hyperlipoproteinemia. It shall be used in combination with drugs which can reduce blood triglyceride such as clofibrate or nicotinic acid for the treatment of type Ⅱb hyperlipoproteinemia. For hyperlipoproteinemia Ⅲ, Ⅳ, Ⅴ, we should not use cholestyramine. In addition, the product can also be used for the treatment of skin itching caused by atherosclerosis and cirrhosis, cholelithiasis.
Cholestyramine: it is mainly used for the treatment of type Ⅱa hyperlipoproteinemia, especially in familial hypercholesterolemia. Also it is used for the treatment of primary biliary cirrhosis, drug-induced cholestatic jaundice itching, hypercholesterolemia, chronic cholecystitis, gallstones, Porphyrin thesaurismosis. Treatment of Atherosclerosis: 4~5g, 3 times/d. Itching: Start amount of 6~10g/d, maintenance dose of 3g/d, administration for 3 times. Long-term use can reduce intestinal binding bile salt, and cause fat malabsorption, long-term use should add vitamin A, D, K and other fat-soluble vitamins and calcium appropriately, gastrointestinal responders may be appropriate with stomach medication.
Product detail pictures:
Related Product Guide:
2021 China New Design Cisatracurium Besylate - Cholestyramine CAS 11041-12-6 USP Standard High Purity – Ruifu , The product will supply to all over the world, such as: , , ,
-

Factory Cheap Hot Imatinib Mesylate Intermediat...
-
Discount Price (S)-(-)-Propylene Carbonate - L...
-

Wholesale Dealers of R-Benzyl Glycidyl Ether -...
-

Professional China Favipiravir Intermediate - ...
-

Factory For Cytarabine - 4-Methyl-3-Nitrobenzo...
-

Wholesale Price China 2 3-Di-O-acetyl-5-deoxy-5...
