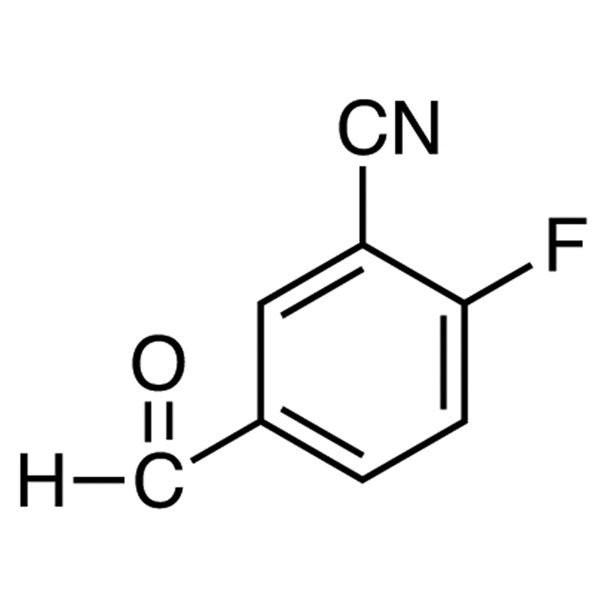
2-Fluoro-5-Formylbenzonitrile CAS 218301-22-5 Puritas ≥98.0% Olaparib Factory intermedium
Alta Puritas, Commercial Productio
Olaparib et intermediis Related:
Olaparib CAS 763113-22-0
2-Fluoro-5-Formylbenzonitrile CAS 218301-22-5
2-Fluoro-5-((4-oxo-3,4-dihydrophthalazin-1-yl) methyl) acidum benzoicum CAS 763114-26-7
1-(Cyclopropylcarbonyl) piperazinum hydrochloridum CAS 1021298-67-8
3-Oxo-1,3-Dihydroisobenzofuran-1-Ylphosphonicum Acidum CAS 61260-15-9
| Nomen chemicum | 2-Fluoro-5-Formylbenzonitrile |
| Synonyma | 3-Cyano-4-Fluorobenzaldehyde |
| CAS Number | 218301-22-5 |
| CATTUS Number | RF-PI451 |
| Stock Status | In Stock, Productio Ascendite ad Tons |
| Formulae hypotheticae | C8H4FNO |
| M. Pondus | 149.12 |
| Liquescens punctum | 80,0 ad 84.0℃. |
| Solubilitas | Solutum in Methanol |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Aspectus | Yellow ad Off-alba pulveris |
| Puritas | ≥98.0% |
| Humor (KF) | ≤0.50% |
| Totalis immunditias | ≤2.0% |
| Test Standard | Enterprise Standard |
| Consuetudinem | Intermedium Olaparib (CAS: 763113-22-0) PARP-Inhibitor |
sarcina: Utrem, aluminium foil, pera, cardboard Drum, 25kg/Drum, vel secundum exigentiam emptoris.
Repono Condition:Repone in vasis signatis in loco frigido et sicco;A luce et humore protege.


2-Fluoro-5-Formylbenzonitrile (CAS: 218301-22-5) adhibetur in praeparatione compositorum heterocyclicorum sicut PARP inhibitores ad usum medicinae.2-Fluoro-5-Formylbenzonitrile pro Olaparib intermedio ponitur (CAS: 763113-22-0).Moleculum parvum inhibitor of PARP1/PARP2 (IC50: 5/1 nM) est, sed minus efficax contra PARP tankyras-1 (IC50: 1.5 µM).Olaparib (AZD-2281, nomen artis Lynparza) est FDA probata therapia pro cancro, a KuDOS pharmaceutica evoluta et postea ab AstraZeneca.Inhibitoris PARP est, polymerasium (PARP) inhibens poly ADP ribosum (PARP), enzyme in DNA reparatione implicatum. Contra carcinomata in hominibus cum hereditariis BRCA1 vel BRCA2 mutationibus agit, quae nonnulla includunt carcinomata ovarii, pectus et prostatae.Mense Decembri 2014, olaparib usus probatus est ut unum agens ab EMA et FDA.FDA approbatio est in causa germline BRCA mutata (gBRCAm) cancri ovarii antecedens, qui tres vel plures priores chemotherapy lineas accepit.