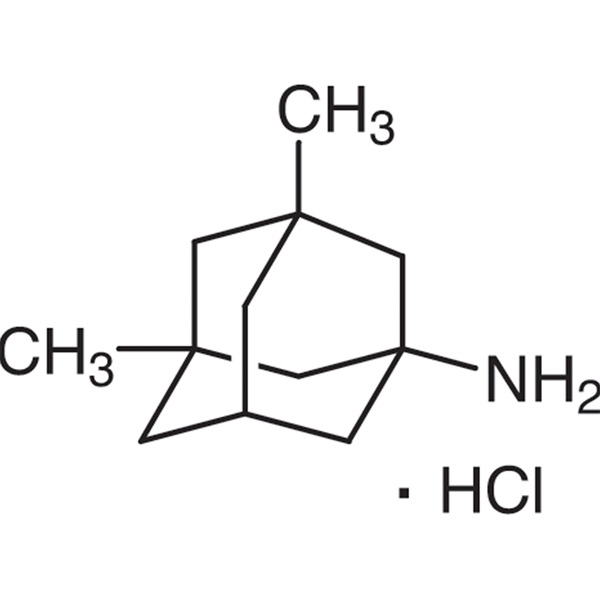
Memantine Hydrochloride Memantine HCl CAS 41100-52-1
Manufacturer cum High puritate et stabulo Quality
Nomen chemicum: Memantine hydrochloride
CAS: 41100-52-1
Hydrochloridis Memantine in curatione morbi praecox
API USP Standard, High Quality, Commercial Production
| Nomen chemicum | Memantine Hydrochloride |
| Synonyma | Memantine HCl;3,5-Dimethyl-1-adamantanamine Hydrochloride |
| CAS Number | 41100-52-1 |
| CATTUS Number | RF-API43 |
| Stock Status | In Stock, Productio Ascendite ad Tons |
| Formulae hypotheticae | C12H22ClN |
| M. Pondus | 215.76 |
| Liquescens punctum | 292℃ |
| Shipping Condition | Sub Ambient Temperature |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Aspectus | Alba vel Off-White Crystalli pulveris |
| Lepidium sativum | IR |
| Damnum in Siccatio | ≤0.50% |
| Metalla gravia (Pb) | ≤10ppm |
| Substantiae cognatae | |
| 1-Methyladamantane | ≤0.30% (CAS 768-91-2) |
| 1,3,5-Trimethyladamantan | ≤0.30% (CAS 707-35-7) |
| Quis incognita immunditia | ≤0.10% |
| Totalis immunditias | ≤0.50% |
| Residua solvens | Ethanol ≤0.05% |
| Residua solvens | Ethylacetate ≤0.05% |
| Contaminatio Microbiologica | Max 5* 102 aerobes et fungi per 1g* Non Escherichia coli |
| Magnitudinem particulis distribuit | Transi 100um |
| pH | 4.5~6.5 |
| Assay | 99.0% ~ 101.0% (ex exaruit basis) |
| Test Standard | USP Standard |
| Consuetudinem | Active Pharmaceutical Ingredient (API);Alzheimer's Morbus |
sarcina: Utrem, aluminium foil, pera, cardboard tympanum, 25kg/Drum, vel secundum exigentiam emptoris.
Repono Condition:Repone in vasis signatis in loco frigido et sicco;Protege a lumine, humore et pestis infestatione.


Shanghai Ruifu Chemical Co., Ltd. est primarium fabricae et supplementi Hydrochloridis Memantini (CAS: 41100-52-1) cum qualitate alta.
Hydrochloridum memantinum (CAS: 41100-52-1) notum est medicamento neuroprotectivo adhibito ad curationem Morborum Alzheimer.Primum medicamentum neuroprotectivum creditur, quod approbationem clinicam FDA et Europaeam a US consequi potest.
Hydrochloridis Memantine, NMDA receptor antagonista, cooperatus est a Laboratorio Forest cum Merz pharmaceuticis et venales sub nomine artis Namendae ad curationem morbi Alzheimer in US post approbationem mense Octobri, 2003. Hoc medicamentum in multis Europaeis praesto fuit. et Asia mercatus ante questus approbationem in US.
-

Memantine Hydrochloridum Memantine HCl CAS 41100...
-

CAS 665-66-7 Intende 98.5%~ 101.5% API
-

Rimantadine Hydrochloride CAS 1501-84-4 Puritas ...
-

1-Bromo-3,5-Dimethyladamantane CAS 941-37-7 Pur...
-

1,3-Adamantanediol CAS 5001-18-3 Puritas >99.0% ...
-

1,3-Dibromoadamantane CAS 876-53-9 Puritas >99.0...
-

1,3-Dimethyladamantane CAS 702-79-4 Puritas >99....
-

1-Acetamidoadamantane CAS 880-52-4 Puritas >99.0...
-

1-Adamantaneacetic Acidum CAS 4942-47-6 Puritas >9...
-

1-Adamantanecarbonyl Chloride CAS 2094-72-6 Pur...
-

1-Adamantanemethanol CAS 770-71-8 Puritas >99.0%...
-

1-Adamantanethanol CAS 6240-11-5 Munditia >98.0%...
-

1-Adamantanecarboxylic Acidum CAS 828-51-3 Puritas...
-

3-Bromodamantane-1-Carboxylic Acidum CAS 21816-0...
-

3-Hydroxy-1-Adamantanecarboxylic Acidum CAS 42711...
-

Adamantane CAS 281-23-2 Puritas >99.0% (GC)