Valsartan CAS 137862-53-4 Intende 98.0~ 102.0% API
Manufacturer Supple Valsartan et intermedias Related:
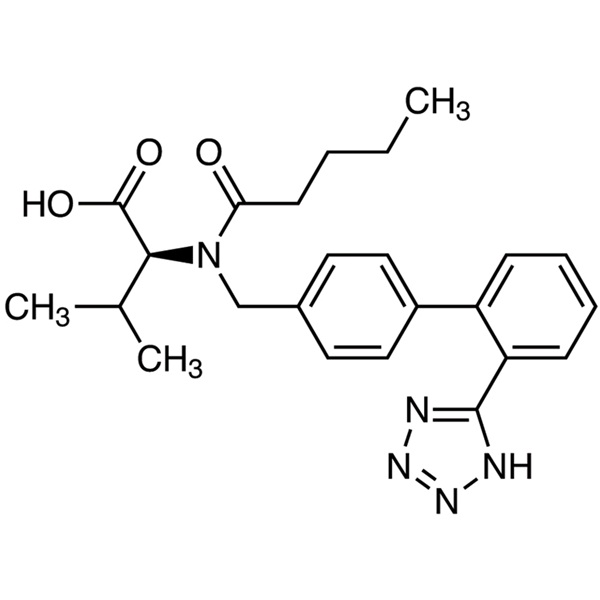
Valsartan CAS: 137862-53-4
L-Valine Methyl Ester hydrochloridum (H-Val-OMe·HCl) CAS: 6306-52-1
| Nomen chemicum | Valsartan |
| Synonyma | N-Valeryl-N-[2'-(1H-tetrazol-5-yl) biphenyl-4-ylmethyl]-L-valine |
| CAS Number | 137862-53-4 |
| CATTUS Number | RF-API32 |
| Stock Status | In Stock, Productio Ascendite ad Tons |
| Formulae hypotheticae | C24H29N5O3 |
| M. Pondus | 435.52 |
| Liquescens punctum | 116.0~117.0℃ |
| Stabilitas | Hygroscopic |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Aspectus | Pulvis albus vel Off-White;Odorless, Hygroscopic |
| Lepidium sativum | IR spectrum specimen aequet vexillum spectrum referat |
| Lepidium sativum | Retentionis tempus maioris culminis in praeparatione chromatogrammi primordium correspondet praeparationi vexillum, prout in Assay obtinetur. |
| Solubilitas | Solutum in Methanol et Ethanol, Parce solubile in Ethylacetate, Leviter solutum in Dichloromethane, in aqua Insolubile. |
| Humor (KF) | ≤1.00% |
| Absorbance (420mm) | ≤0.02% (λ=420nm, C=0.05g/ml, L=1cm) |
| Residere in Ignition | ≤0.10% |
| Metalla gravis | ≤10ppm |
| D-Valsartan | ≤1.00% (HPLC) |
| Substantiae cognatae (HPLC) | |
| Butyryl-Valsartan | ≤0.20% |
| Benzyl-Valsartan | ≤0.10% |
| Uno Antonio Inpurity | ≤0.10% |
| Totalis immunditias | ≤0.30% (Exclusis D-Valsartan) |
| RELICTUM Solvents (GC) | |
| Methanol | ≤3000ppm |
| Ethyl Acetate | ≤5000ppm |
| N,N-Dimethylformamide | ≤880ppm |
| Toluene | ≤890ppm |
| Puritas / Analysis Methodus | > 99.0% (HPLC calculata anhydrous, libero fundamento solvendo) |
| Test Standard | Pharmacopoeia Civitatum Foederatarum (USP) |
| Consuetudinem | Active Pharmaceutical Ingredient (API) |
Valsartan (CAS: 137862-53-4) Processus Flow
sarcina: Utrem, aluminium foil, sacculum, 25kg/cardboard Drum, vel secundum exigentiam emptoris
Repono Condition:Repone in vasis signatis in loco frigido et sicco;Protege a lumine et humore


Shanghai Ruifu Chemical Co., Ltd. est praecipuum fabricam et supplementum Valsartanae (CAS: 137862-53-4) magna qualitate.Valsartan est angiotensin nonpeptide II AT1 receptor adversarius, antihypertensivus, potentiam habet ad pressuram sanguinis et cordis defectum investigationis altae.
Valsartani effectus antihypertensivos fortior quam enalapril sunt et ad hypertensionem tractandum apta est, lenis ad hypertensionem primariam moderandam, et praesertim hypertensionem secundariam ex renum damno causatam.Signanter reducere proteinuria potest aegros hypertensiones cum diabete vel functionibus normalibus iecoris, et acidum uricum et sodium urinarium ad renem tuendam promovere potest.Valsartan etiam convenit ad mortalitatem cardiovascularem reducendam in magno periculo aegros (defectum ventriculi sinistri vel dysfunctionis) postquam cordis impetum experitur.
-

Valsartan CAS 137862-53-4 Intende 98.0~ 102.0% API
-

Triphenylvalsartan CAS 7693-46-1 Munditia >97.0% ...
-

L-Valine Methyl Ester hydrochloridum (H-Val-OMe·...
-

Cinchonidine CAS 485-71-2 Percipe 98.5%~101.0% AP...
-

Bortezomib CAS 179324-69-7 Munditia ≥99.0% (HPLC).
-

Darunavir CAS 206361-99-1 Anti-HIV Puritas ≥99.0...
-

Esmolol Hydrochloridis CAS 81161-17-3 Puritas ≥99...
-

Fingolimod Hydrochloridis CAS 162359-56-0 Puritas...
-

Lasofoxifene Tartrate CAS 190791-29-8 Chiral Pu...
-

Iurasidone hydrochloride CAS 367514-88-3 Puritas...
-

Memantine Hydrochloridum Memantine HCl CAS 41100...
-

Rapamycin Sirolimus RAPA CAS 53123-88-9 Intende ≥...
-

Tacrolimus FK-506 Fujimycin CAS 104987-11-3 API...
-

Revaprazan Hydrochloride CAS 178307-42-1 Assay ...
-

Tobramycin Sulfate CAS 49842-07-1 Potentia 634μg...
-

Ezetimibe CAS 163222-33-1 Puritas 98.5%~ 102.0% (...