Valsartan CAS 137862-53-4 Assay 98.0~102.0% API
Manufacturer Supply Valsartan and Related Intermediates:
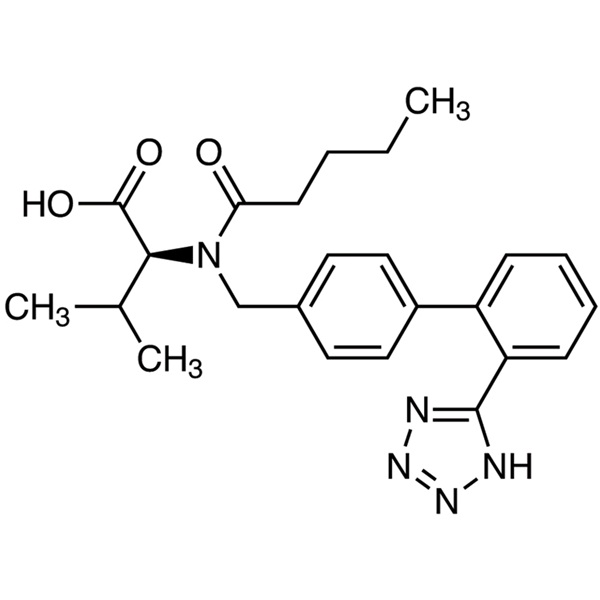
Valsartan CAS: 137862-53-4
L-Valine Methyl Ester Hydrochloride (H-Val-OMe·HCl) CAS: 6306-52-1
| Chemical Name | Valsartan |
| Synonyms | N-Valeryl-N-[2'-(1H-tetrazol-5-yl)biphenyl-4-ylmethyl]-L-valine |
| CAS Number | 137862-53-4 |
| CAT Number | RF-API32 |
| Stock Status | In Stock, Production Scale Up to Tons |
| Molecular Formula | C24H29N5O3 |
| Molecular Weight | 435.52 |
| Melting Point | 116.0~117.0℃ |
| Stability | Hygroscopic |
| Brand | Ruifu Chemical |
| Item | Specifications |
| Appearance | White or Off-White Powder; Odorless, Hygroscopic |
| Identification | IR spectrum of the sample matches the reference standard spectrum |
| Identification | The retention time of the major peak in the chromatogram of the assay preparation corresponds to that of the standard preparation, as obtained in the Assay. |
| Solubility | Soluble in Methanol and Ethanol, Sparingly Soluble in Ethylacetate, Slightly Soluble in Dichloromethane, Insoluble in Water |
| Moisture (K.F) | ≤1.00% |
| Absorbance (420mm) | ≤0.02% (λ=420nm, C=0.05g/ml, L=1cm) |
| Residue on Ignition | ≤0.10% |
| Heavy Metals | ≤10ppm |
| D-Valsartan | ≤1.00% (HPLC) |
| Related Substances (HPLC) | |
| Butyryl-Valsartan | ≤0.20% |
| Benzyl-Valsartan | ≤0.10% |
| Single Unknown Inpurity | ≤0.10% |
| Total Impurities | ≤0.30% (Excluding D-Valsartan) |
| Residual Solvents (GC) | |
| Methanol | ≤3000ppm |
| Ethyl Acetate | ≤5000ppm |
| N,N-Dimethylformamide | ≤880ppm |
| Toluene | ≤890ppm |
| Purity / Analysis Method | >99.0% (HPLC calculated on anhydrous, solvent-free basis) |
| Test Standard | United States Pharmacopoeia (USP) |
| Usage | Active Pharmaceutical Ingredient (API) |
Valsartan (CAS: 137862-53-4) The Production Process Flow
Package: Bottle, Aluminum foil bag, 25kg/Cardboard Drum, or according to customer's requirement
Storage Condition: Store in sealed containers at cool and dry place; Protect from light and moisture


Shanghai Ruifu Chemical Co., Ltd. is the leading manufacturer and supplier of Valsartan (CAS: 137862-53-4) with high quality. Valsartan is a nonpeptide angiotensin II AT1 receptor antagonist, Antihypertensive, has the potential for high blood pressure and heart failure research.
Valsartan’s antihypertensive effects are stronger than those of enalapril and is suitable for treating hypertension, mild to moderate primary hypertension, and especially secondary hypertension caused by renal damage. It can significantly reduce proteinuria for hypertension patients with diabetes or normal liver functions, and it can promote uric acid and urinary sodium to protect the kidney. Valsartan is also suitable for reducing the cardiovascular mortality for high risk patients (left ventricular failure or dysfunction) after experiencing a heart attack.
-

Valsartan CAS 137862-53-4 Assay 98.0~102.0% API
-

Triphenylvalsartan CAS 7693-46-1 Purity >97.0% ...
-

L-Valine Methyl Ester Hydrochloride (H-Val-OMe·...
-

Cinchonidine CAS 485-71-2 Assay 98.5%~101.0% AP...
-

Bortezomib CAS 179324-69-7 Purity ≥99.0% (HPLC)...
-

Darunavir CAS 206361-99-1 Anti-HIV Purity ≥99.0...
-

Esmolol Hydrochloride CAS 81161-17-3 Purity ≥99...
-

Fingolimod Hydrochloride CAS 162359-56-0 Purity...
-

Lasofoxifene Tartrate CAS 190791-29-8 Chiral Pu...
-

Lurasidone Hydrochloride CAS 367514-88-3 Purity...
-

Memantine Hydrochloride Memantine HCl CAS 41100...
-

Rapamycin Sirolimus RAPA CAS 53123-88-9 Assay ≥...
-

Tacrolimus FK-506 Fujimycin CAS 104987-11-3 API...
-

Revaprazan Hydrochloride CAS 178307-42-1 Assay ...
-

Tobramycin Sulfate CAS 49842-07-1 Potency 634μg...
-

Ezetimibe CAS 163222-33-1 Purity 98.5%~102.0% (...